
Yvonne was an active mother and member of her faith community before her venous leg ulcer became debilitating and she had to be admitted to a hospital. Because of Apligraf, she was able to heal in around two weeks. Watch her testimonial and more by following the link below.

Organogenesis Inc:
Apligraf and Dermagraft

Integra LifeSciences Corporation
Joseph Vacanti & Charles Vacanti
Popularizing TE
Tissue engineering was thrown into the public view when Joseph Vacanti and his collegues wrote a ground-breaking paper in the journal Plastic Reconstructive Surgery. Later, Dr. Vacanti and Dr. Robert Langer wrote a paper describing the technology of tissue engineering; it is often referenced as the beginning of the new biomedical discipline of TE.
The Vacanti Mouse
The picture adjacent grew to have great controversiality, mis-labeled by anti-genetics groups as a "genetically engineered mouse;" in actuality it was a tissue engineering project by Dr. Charles Vacanti (the brother of Joseph), using a scaffold made of the same material as dissolving surgical stitches. The scaffold was seeded with cartilage cells from a cow's knee, and then was implanted into the mouse between the skin and muscle layers.





"Like a kid in a candy store, overcome by the sheer number of interesting medical problems that might yield to his engineering know-how and imagination"
- Dr. Robert Langer on his first position as a young chemical engineer working at Harvard University under Judah Folkman
Called the "Most Cited Engineer in History," Dr. Robert Langer has written over 1,300 articles, and holds more than 220 major awards (see a list of his accomplishments on the right). He is regarded as the founder of tissue engineering in regenerative medicine, creating engineered blood vessels and muscle that is vascularized (populated with blood vessels).
Dr. Robert Langer
MEET THE TISSUE ENGINEERS: STORIES OF COLLABORATION, FAILURE, AND ULTIMATELY, SUCCESS...
Innovators: Individuals Making a Difference

Harvard Medical School, Joseph and Charles Vacanti
The "Vacanti" mouse, shown here in 1995 at its home at the University of Massachusetts.
This mouse has become a symbol of all of the public misconceptions and fear surrounding tissue engineering.
Dr. Joseph Vacanti
Dr. Charles Vacanti
This work by both brothers was important not only because of the discoveries that they prompted, but also by the enormous publicity that such a sensational idea was able to generate. Tissue engineering had grown from an obscure topic barely mentioned in papers to a controversial issue at the forefront of public debate.
Massachusetts Institute of Technology And
Dr. Ioannis Yannas
With Regenerated Skin, the victim's skin cells move into the 'template' that the collagen provides. As the template is broken down and absorbed, the cells form a new dermis.
With normal burns, the wound retracts and forms a scar; neither the epidermis (top level) nor the dermis (lower level) is able to regenerate.
"Skin...not scar"
In 1969, MIT professor Ioannis Yannas found a "mutual clicking" in Dr. John Burke, the head of the Shriners Institute in Boston. Burke's patients were mostly children with near-fatal burns, with too little remaining skin to graft and too little time to wait, and when Dr. Yannas was given a tour, he was 'shocked beyond belief'; he states 'there was a great deal of human misery confronting me...and I felt that I had to do something about it".
These images stayed with Yannas all of his life, driving him to try and try again to find some way to speed up the closing of the horrific wounds.

When Dr. Yannas tried collagen, it seemed to slow down the rate of wound closure. It took some time before they realized: the collagen dressings had not made scars: they had made skin (see P7.1 and P7.2). The children were able to regrow the skin that they had lost; befor dissolving, the collagen served as a scaffold onto which new skin - even the lower dermis level - was able to grow. Since then, "Regenerated Skin" has been saving the lives of burn victims around the world.

Other tissue engineering endeavors include finding ways to apply regenerative medicine to war injuries. In 2013, the Institute was selected to lead a $75 million federally-funded project combining the work of 30+ academic institutions; the project is focusing on healing severe limb, facial, and skull injuries, as well as skin remedies such as regeneration and preventing rejection of grafts.
Wake Forest Institute for Regenerative Medicine - Dr. Anthony Atala
The Wake Forest Institute for Regenerative Medicine is internationally known as the very first institution able to successfully implant laboratory-grown organs into humans. With the expertise of Dr. Anthony Atala, the director of the Institute, Wake Forest has been able to save the lives of many children suffering from spina bifida (a spinal cord abnormality) which paralyzed their bladders. Atala harvested stem cells from patients' bladders, seeded them onto bladder-shaped scaffolds, and after two months was able to implant them into the patients, where they subsequently attracted their own blood vessels and allowed the children to lead normal, free lives.


A researcher at Wake Forest University dips human bladder cells - seeded onto a bladder-shaped scaffold - into a growth solution.

An ear scaffold, seeded with cells, rests in a growth serum at the Wake Forest Institute.
One of the Wake Institute's 3-D printed ear scaffolds.
Dr. Atala and a few members of his team describe the steps they are taking to help the wounded warriors.
In the future, Dr. Atala and his team hope to advance their experimental 3-D printing of advanced organs (see the video below) and scaffolds (image I-1 shows a 3-D printed ear scaffold, and image I-2 shows the scaffold seeded with cells and resting in a growth serum).
: Dr. Ioannis Yannas
Wake Forest School of Medicine and Dr. Anthony Atala
Massachusetts Institute of Technology: Dr. Langer
There are two levels to this innovative skin wound care device. 1) The first layer is made of
silicone, and it functions as
the skin's epidermis as it
heals, protecting it from
temperature loss,
moisture, and infection.
2) The second layer is a matrix
composed of collagen
from bovine tendons into
which new dermal (lower
skin level) cells can
repopulate.
Integra Bilayer Wound Matrix



Both Apligraf and Dermagraft treat diabetic foot ulcers and venous leg ulcers. They actively manage wounds by delivering living cells, proteins, and collagen to the injury sites. A study on Apligraf found that since it is a living cell solution, it is able to heal 30% to 50% more wounds, in 1/3 less time.
These products provides great relief especially to diabetics whose circulation problems lead to chronic ulcers, which could lead to infection and amputation if left as-is, unable to heal.
Healing Foot Ulcers
Companies: Selling TE Solutions
An animation showing the steps of Dermagraft production. The video walks through how the cells are seeded onto the scaffold, then grow, secreting growth factors, proteins, and more: this product is filled with metabolically active human cells, which is why it can heal so much more effectively.

This product was the first and remains the only FDA approved biological product for treating articular (knee) cartilage injuries.
A biopsy is taken of the patient's chondrocytes, and 3 to 5 weeks later the culture has grown to include 12-48 million cells before it is implanted into the patient.
As with most TE solutions, this one poses little risk of disease transmission, since it has been grown from the patient's own cells.
Carticel: Replacing Damaged Knee Cartilage
Genzyme: A Sanofi Company

A cartilage injury such as one that could be treated with Carticel.
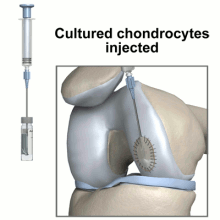
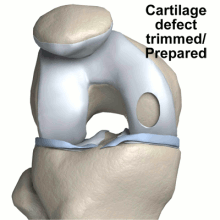
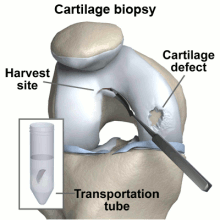
The diagrams below illustrate steps to Carticel treatment, starting with harvesting cells for a biopsy, then preparing the defect for the injection of the chondrocytes, now multiplied into millions of cells.
A diagram showing how Provenge treatments are performed, collecting cells and then making them into the patient's specifically-made dose.
Dendreon's Provenge
Provenge uses tissue engineering to reprogram a patient's own immune cells to fight their cancer: specifically, advanced prostate cancer.
Often touted as a major breakthrough technology, it reduced the death of patients by 22.5% in its clinical trial. These ACI (active cellular immunotherapy) solutions have been able to reach the commercial level, generating about $72 million in sales in 2012, only two years after its approval by the FDA.
Provenge: Reprogramming Immune Cells



Innovator Images:
Company Logos:








